| Company Name: | Chengdu RunZeBenTu Chemical Co., Ltd Gold |
| Tel: | 13096311329 028-88469284 qq:616445927 |
| Email: | 616445927@qq.com |
| Products Intro: |
Product Name:Formic acid
CAS:64-18-6
Package:5g/25g/100g/250g/500g |
| Company Name: | Shanghai Macklin Biochemical Co.,Ltd. Gold |
| Tel: | 021-51821861 |
| Email: | sales@macklin.cn |
| Products Intro: |
Product Name:Formic acid solution
CAS:64-18-6
Purity:for HPLC, 50% in water, 49-51% (T) Package:500ml |
|
| | Formic acid Chemical Properties |
| mp | 8.2-8.4 °C(lit.)
| | bp | 101 °C | | density | 1.22 | | vapor density | 1.03 (vs air)
| | vapor pressure | 52 mm Hg ( 37 °C)
| | refractive index | n20/D 1.377
| | FEMA | 2487 | | Fp | 133 °F
| | storage temp. | 2-8°C
| | Water Solubility | MISCIBLE | | Sensitive | Hygroscopic | | Merck | 14,4241 | | BRN | 1209246 | | Stability: | Stable. Substances to be avoided include strong bases, strong oxidizing agents and powdered metals, furfuryl alcohol. Combustible. Hygroscopic. Pressure may build up in tightly closed bottles, so bottles should be opened carefully and vented periodically. | | CAS DataBase Reference | 64-18-6(CAS DataBase Reference) | | NIST Chemistry Reference | Formic acid(64-18-6) | | EPA Substance Registry System | Formic acid(64-18-6) |
| | Formic acid Usage And Synthesis |
| Important chemical raw materials | Formic acid is an important chemical raw material. In 1670 Fisher first discovered. In1749, A.S.Marggret first makes preparation of pure acid. Discovered because of the first distillation red ants, named formic acid. Formic acid widely exist in nature, such as red ant, bee, caterpillars and other secretions, plant leaves and roots, and fruit. The simplest carboxylic acid, compared with other fatty acid, structure is more special, strong acid. It is a colorless transparent stimulation smoke odor liquid, molecular formula is HCOOH. The molecular mass is 46.03. The relative density is 1.2196. The melting point is 8.4℃, the freezing point is 7.℃, the boiling point is 100.7℃, 50 ℃ (15.999 ×103Pa),. Refractive index is 1.3714. flash point is 68.9℃. Viscosity is1.784mPa.S. ignition point is 410 ℃, miscible with water, ethanol, ethyl ether. Formation of high boiling point binary azeotrope with water, containing 77.5% of the goods, azeotropic point is 107.3 ℃, the boiling point is higher than the boiling point of water and pure acid. The carbonyl group directly with hydrogen connected formate has both characteristics of acid and aldehyde some properties. It can form salt and ester; can react with amine and form amid and unsaturated hydrocarbon addition, produce ester. Aldehyde reduction of embodies the nature, such as the silver nitrate ammonia solution (Toulon reagent) reduction to metallic silver, the potassium permanganate fading, so that sulfur dioxide reduction for thiosulfate ion (SO2, S2O32-), the mercury nitrate is reduction of mercury. Formic acid and sulfuric acid can be dehydrated to produce carbon monoxide. Heated to 160 ℃ can also generate decarboxylation, carbon dioxide and hydrogen; In the condition of copper - chromium, platinum, palladium catalyst, It can also produce decomposition reaction to carbon dioxide and hydrogen generation. This product is very corrosive and irritating, inhalation of vapor, the respiratory irritation and inflammation; stick to the skin, make the skin blistering. Chronic poisoning, resulting in hematuria. Rat of LD50 is1210mg/kg by oral. The workplace maximum allowable concentration is 5×10-6. The storage should be away from fire, heat, and oxidant, alkali, acid, H foaming agent and cyanide isolation. When loading and unloading to handle gently and not inverted. Prevent from the water, not to breakage of packing. For the leakage of material, it need to be rinsed with water, but when you wear a protective tool. Reaction in contact with the skin, rinse immediately with plenty of water, if necessary, apply ointment. Inhalation poisoning, emergency to hospital.
This information is edited by the Chemicalbook Han Ya.
| | The simplest carboxylic acid | Formic acid is the simplest carboxylic acid, was first obtained by distillation of red ants, it is also called formic acid.
Formic acid are found in ants, secretions of bees and caterpillar. Being stung skin irritation, itching, is due to the stimulatory effect of bee needle release of formic acid. Formic acid is also present in such as nettle, scorpion grass, pine needles, and some fruit (such as green grapes) and the muscles of the human body, blood fluid and excretion.
Formic acid is a fatty acid only connected with hydrogen atoms in carboxyl and hydrogen atom denounced the electronic power is far less than that of alkyl, the electron density of carboxyl carbon atom is lower than that of the other carboxylic acid, and conjugation effect and carboxyl oxygen atom electronic prefer carbon, so The acidity of formic acid is higher than in the same series of other carboxylic acid.
The structure of formic acid can be regarded as combined aldehydes with hydroxyl radical, so the formic acid molecules containing aldehyde and aldehyde has some properties, such as reduction of silver ammonia solution to form silver mirror. It can fade the potassium permanganate solution, these reactions can be used for the qualitative identification of formic acid. Formic acid has the nature of the general acid, condensation reaction of aldehydes.
Formic acid for molecules containing aldehyde group is reductive, in the textile, printing and dyeing industry is used acid reductant bleaching straw hat, leather and clothes on the ink spots and rust removal. It can make rubber slurry condensation of raw rubber, in the tanning industry is used do lactic acid coagulant is a rubber coagulants. Formic acid is raw material for preparation of oxalic acid, sodium formate heating to 360 DEG C, to produce hydrogen oxalate. Formic acid or dyeing, mordant, metal surface processing agent and antiseptic.
| | The purification of formic acid | Formic acid as a reducing agent, can be used for the determination of arsenic, bismuth and other elements, can be used as organic and inorganic solvents.
Process for the purification of formic acid:
1. atmospheric distillation
500ml of analytical pure formic acid in 1000ml distilled bottle, glycerin bath heating, bath temperature control below 110℃, with atmospheric distillation abandoned to the previous distillate 50ml, collected in the middle distillate 350 ~ 400ml.
2. mixed distillation
(1) formic acid ester were mixed with distilled and distilled liquid points for two-phase, then the propyl formate phase distillation to obtain the pure acid, another phase is an aqueous phase containing 1% formic acid.
(2)when distilled to formic acid solution to add the tertiary amine, quinolone , dimethylaniline and other tertiary amine mixed distillation, the water phase first steam out, give up, continue to distillation, then added amine and formic acid can be separated.
Reference materials: Wu Xinyou, Yuan Shengquan, Zhai Jinxi. Handbook of purification and preparation of analytical reagents. Beijing: Metallurgical Industry Press, 2000. | | The preparation method | In 1896, European countries use sodium hydroxide and carbon monoxide direct reaction to the production of sodium formate, acid hydrolysis acid, and put into industrial production.
In 1980, the United States scientific design company and other successful development of methanol to produce formic acid, an annual output of 20 thousand tons.
Process for the production of formic acid with carbon monoxide and powdered sodium hydroxide is as follows:
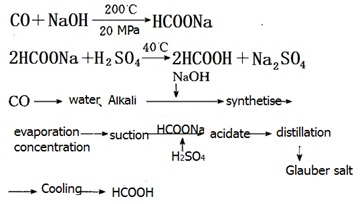
| | Applications in leather industry | 1. Formic acid as monocarboxylic acid is the strongest acid, having loose on the collagen fibers but more gently than inorganic acids, uniform. For fur processing of acid leaching, with destructive little of mildew, soft effect, fiber dispersion of the fine and uniform, finished product has soft, plump characteristics.
2. In chrome tanning, formic acid can play a masking effect, the masking effect is stronger than acetic acid.
3. Formic acid is a strong reducing agent, and it is easy to be oxidized and decomposed.
4. Formic acid dyeing and fixing effect of acid dyes, and can make the leather color bright-coloured.
5. Fur soaking aid, with reduced water immersion liquid pH value (5 ~ 5.5), to promote the growth of the skin and the expansion of water, inhibit the growth of bacteria, formic acid, acetic acid and other organic acids additives are also commonly used in hair loose or not easy to soak the fur.
6. Acid can be used as a pickling material, can also be mixed with sulfuric acid for pickling.
7. Rapid plant pickling material tanning process, accelerate the penetration of tannin.
8. The preparation of sodium formate masking agent. The best ratio of sodium formate for chrome tanning masking for formic acid: 1mol sodium than 1mol basic chromium sulfate.
9. Neutralizer one. In the furniture leather production, and adding 1% ~ 2% of sodium formate, is help to neutralize and deeper, keep grain surface smooth.
10. Regulation of the dye bath pH value, solid color.
11. Leather brush with the last brush dye plus 1~ 2g/L formic acid to solid color.
12. Reduced water hardness: 100kg water to join 3.2mL50% formic acid (relative density of 1.121), can reduce the hardness of a degree.
13.Supplement in the production of renewable leather, with the role of promoting cohesion, dosage in the following (concentration) is appropriate.
14. White leather rinse the whitening effect than potassium permanganate, Hyperion, but less than oxalic acid.
| | Toxicity and protection | Formic acid is liquid with the penetration and pungent odor, is an organic acid corrosive materials. It is with the dissolution of fat, so the skin can absorb it, The stimulation of skin and mucous membranes is stronger than that of acetic acid. The steam has strong stimulation to eyes, liquid can make skin redness, foaming and local gangrene. It is the similar inorganic acid corrosive on mucosa. Formic acid vapor can cause sore throat, cough and chest pain etc. stimulate symptoms.
Inhalation poisoning should be immediately from the scene, a breath of fresh air, 2% sodium hydrogen carbonate atomization inhalation. Burnings washed through flush with plenty of water, and then 2~ 4% sodium hydrogen carbonate solution washing, serious persons please occupational disease hospital for treatment.
The highest allowable concentration of formic acid in the air is 5ppm (9mg/m3), also can be used as a preservative for cosmetics, the maximum permissible concentration of 0.5% (acid).
| | Maximum allowable amount and maximum allowable residual standard of food additives | 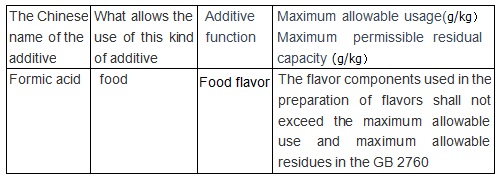
| | Chemical property | It is colorless flammable liquid, with a strong pungent odor. And it is soluble in water, ethanol and ether, slightly soluble in benzene.
| | Application | 1. Used for the preparation of formate, formic acid ester, formamide and so on, also used in medicine, printing and dyeing, dyes, leather and other industries.
2. Used as chemical reagent.
3. Used as a reducing agent, buffering agent, also used for pesticide preparation.
4. used as green feed additives.
5. Used in the manufacture of pulp sterilization mildew.
6. Flavor enhancer. Mainly used for the preparation of pineapple, rum and smoked flavor. Both it has disinfection antiseptic effect.
7. Formic acid is one of basic organic chemical raw materials, widely used in pesticide, leather, textile, printing and dyeing, pharmaceutical and rubber industry, etc. It also can produce all kinds of solvent, plasticizer, rubber coagulator, animal feed additives and new process for the synthesis of insulin. The consumption of formic acid in China, accounting for about 45% of the pharmaceutical industry, chemical industry accounted for 30%, light industry, textile and other sectors accounted for 25%. Formic acid is one of the important exports of chemical products in our country. At present in our country all adopt the method of sodium formic acid production. Formic acid is a cheap, volatile and less corrosive inorganic acid substitute, widely used in light industry. In the textile and dyeing industry, it is used as eliminating nitrous acid gas agent which is produced by India and submicron sodium nitrate method, weak acid dyes and dyeing auxiliaries neutral dyes, polyamide dyeing reactive dyes. There is no acid residues in the fabric and it is a stronger acid than acetic acid, can make reduction of six mediated chromium, so it can improve the utilization rate of dyes in chrome mordant process. Instead of sulfuric acid with formic acid can avoid the degradation of cellulose, and the acidity was moderate, dyeing and learning, so it is a good dyeing assistant. Formic acid as substitutes for inorganic acid, bleaching, tanning in the shed, and lime and prevent wet leather mildew. With formic acid as coagulator can be made of high quality natural rubber, reduce the production cost, but also can be used for the regeneration of waste rubber. Production of feed additives with formic acid, it has great potential in silage. Formic acid has the function of inhibiting or preventing the growth of mold, the feed can change the nature of the fermentation form, and often add acid to enhance the anti-mold effect. Green feed using formic acid treated cows can prevent to decrease production of milk, in winte,r the fattening effect has significantly improved. In the food industry, it is used in making formic acid Wine industry disinfection, antiseptic, used as canned cleaning disinfectant, fruit juice and food preservative. Formic acid derivatives is medicine, pesticides, dyes, perfumes, intermediate of the solvent , used for producing Borneol, aminopyrine, caffeine, vitamin B1, analgin, Chlordimeform, Triadimefon, Tricyclazole, dimethylformamide. Formic acid derivatives to replace the formamide and formic acid esters in more industrial application (see the entry for formamide derivatives). The use of esters of formic acid in the perfumery industry is very broad, such as: ethyl formate-peach, berries and other fruit flavors; Isoamyl formate-fruit flavor, leather flavor; formic acid ester - apple essence; acid heptyl ester-apricot, plum, peach and other fruit flavor; formic acid decyl ester - Neroli essence iris and oil; benzyl formate- jasmine floral and soap flavor; Formic acid ester- jasmine and tuberose and other flavors; Citronellyl formate-roses, sweet scented osmanthus, wild lily and Ding Xiangzhi acid- carnation essence; geranyl formate-rose, neroli, rose essence etc. linalyl formic- lavender, sweet lemon flavor; Formic acid Mint ester- cosmetics, spray flavor; Phenylethyl Formate- white rose, orchid, chrysanthemum essence; thymol formate-cosmetics perfume. | | Methods of production | 1.Formate sodium method: With 20%-30% sodium hydroxide solution to absorb refining CO gas under condition of 160-200℃ and 1.4-1.8Mpa, react to sodium formate solution. Then we mixed the same amount of formate acid solution and formate acid solution, with the condition of dilute sulfuric acid it react to formic acid and sodium formate, distillation to formic acid and water azeotrope (formate containing approximately 75%),then refined to the product.
2. Methyl formate method: Under the catalysis of sodium methoxide, methanol and CO react to methyl formate in 80℃ and 4MPa. Methyl formate in the presence of acid catalyst, and at 90-140℃ and 0.5-1.8Mpa,is hydrolysis of formic acid and methanol. And can be obtained formic acid by separation, Methanol circulate to use.
3.Methyl ester method. Ammonia methanol solution at 70℃ and 32.5Mpa,absorb CO and generate formamide, which is separated and react with the same amount of 68%-74 of sulfuric acid to formic acid and ammonium sulfate. After Formic acid steam, the product is refined. Raw material consumption quota: methanol 31kg/t, carbon monoxide 702kg/t, ammonia 314kg/t, sulfuric acid 1010kg/t.
4. Obtained by oxidation of methane: at high temperature and high pressure, carbon monoxide and sodium hydroxide react to sodium formate and then decomposition at the condition of sulfuric acid low temperature, after fractionation, it is obtained.
5. Carbon dioxide method: in the presence of palladium complex catalyst, in the triethylamine aqueous solution, carbon dioxide and hydrogen at 140 to 160 ℃ react to Formic acid.
| | Category | corrosives
| | Toxicity grading | poisoning
| | Acute toxicity | Oral administration of LD50: 1100 mg / kg in rats; oral administration of LD50: in mice 700 mg / kg.
| | Stimulus data | Skin - 610 mg for the rabbit, light; eye - 122 mg for the rabbit, severe.
| | Chemical Properties | Clear, colorless liquid | | Explosive hazard characteristics | It can be explosion with air mixed.
| | Combustible hazard characteristics | In the case of high temperature and flame, It can be combustible; In case of hydrogen peroxide,it caused an explosion; combustion generated to stimulate the smoke.
| | Storage and transportation characteristics | Low temperature and dry air drying; separate storage with alkali, oxidant, H agent, cyanide.
| | Fire extinguishing agent | Sand, carbon dioxide, fog water.
| | Professional standards | TLV-TWA 5ppm (9 mg/ m3); STEL 10 ppm (18 mg / m3).
| | General Description | A colorless liquid with a pungent odor. Flash point 156°F. Density 10.2 lb / gal. Corrosive to metals and tissue. | | Air & Water Reactions | Fumes in air. Soluble in water with release of heat. | | Reactivity Profile | Formic acid reacts exothmerically with all bases, both organic (for example, the amines) and inorganic. Reacts with active metals to form gaseous hydrogen and a metal salt. Reacts with cyanide salts to generate gaseous hydrogen cyanide. Reacts with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides to generate flammable or toxic gases. Reacts with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Reacts with carbonates and bicarbonates to generate carbon dioxide but still heat. Can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. May initiate polymerization reactions or catalyze other chemical reactions. A mixture with furfuryl alcohol exploded [Chem. Eng. News 18:72(1940)]. | | Health Hazard | Liquid causes skin and eye burns. Vapors are irritating and painful to breath. Vapor exposure may cause nausea and vomiting. | | Fire Hazard | Special Hazards of Combustion Products: Toxic vapor generated in fires |
| | Formic acid Preparation Products And Raw materials |
| Raw materials | Sodium hydroxide-->Methanol-->Sulfuric acid -->Triethylamine-->Ammonia-->Sodium methanolate-->Phosphorous acid-->CARBON MONOXIDE-->PETROLEUM ETHER-->Sodium formate-->Methyl formate-->METALLURGICAL COKE | | Preparation Products | [4-AMINO-2-(TRIFLUOROMETHYL)PYRIMIDIN-5-YL]METHANOL-->8-BROMO-3-METHYL-3,7-DIHYDRO-PURINE-2,6-DIONE-->Sulfur Yellow GC-->2-Ethylbutyric acid -->3,4-DIHYDROISOQUINOLINE-->Benzyl formate-->N-Methylformamide-->1-METHYLPIPERIDINE-4-CARBOXYLIC ACID HYDROCHLORIDE-->2-METHANESULFONYL-4-METHYL-PYRIMIDINE-->5-Ethyl-2-pyridineethanol-->METHYL PENT-4-YN-2-YLCARBAMATE-->Sulfur Red Brown B3R-->3,7-DIMETHYL-7-OCTEN-1-OL-->5-Methyl-2-(methylsulfonyl)pyrimidine ,97%-->5-AMINO-6-CHLORO-PYRIMIDIN-4-OL-->4-AMINO-2-(TRIFLUOROMETHYL)PYRIMIDINE-5-CARBALDEHYDE-->7-Hydroxy-6-methoxy-3,4-dihydroisoquinoline-->1-METHYL-1-CYCLOHEXANECARBOXYLIC ACID-->(1H-INDAZOL-3-YL)-ACETIC ACID-->Diethoxymethyl acetate-->Epoxidized soya bean oil-->C.I.BASICBLUE22-->dimethyl dodecyl thioic propylene betaine-->5,7-dichlorooxazolo[5,4-d]pyrimidine-->GUANINE SULFATE-->1-FORMYL-4-METHYLPIPERAZINE-->4-Azabenzimidazole-->1,3,5-TRIMETHYL-1,3,5-TRIPHENYLCYCLOTRISILOXANE-->4,4'-Methylenebis(N,N-dimethylaniline)-->3-FLUORO-N-METHYLANILINE-->2-Hydroxy-N-(4-methoxyphenyl)-11H-benzo[a]carbazole-3-carboxamide-->Pigment Yellow 83-->2-AMINO-4,4'-DICHLORODIPHENYL ETHER-->2-OXO-2,3-DIHYDRO-BENZOOXAZOLE-5-CARBOXYLIC ACID-->1-(2-Hydroxyethyl)-4-methylpiperazine-->1H-Benzimidazole-5-carboxylic acid-->3-METHYLTHIOPHENE-2-CARBONITRILE-->Geranyl formate-->ETHYL 2,4-DICHLOROBENZOATE-->CINNAMYL FORMATE |
|